ESSAY
This paper is in two sections, A and B
Answer Question 1 in Section A and any other three questions in Section B.
Credit will be given for clarity of expression and orderly presentation of material
PART I
[40 marks]
Answer all of Question 1
-
-
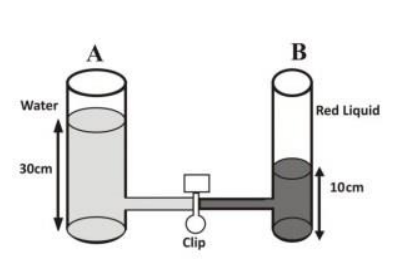
In an experiment, as in the set-up above, two glass containers A and B of different sizes are joined together with a tube and clipped. Water is poured into container A to a height of 30 cm and a red liquid is poured into B to a height of 10 cm. The clip is then removed so that the liquids join together.
-
State two observations that will be made immediately the clip is removed. Explain the observations in (i) What two observations will be made after a long time? Explain.
-
State the observations that will be made about nails 1, 2, 3, and 4.
-
State the observations that will be made about the temperatures recorded by thermometers A, B, C, and D.
-
What mode of heat transfer is demonstrated in the experiment?
-
State one effect of heat that is associated with the experiment.
-
State the aim of the experiment.
-
-
-
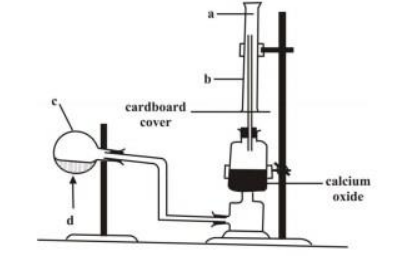
The set-up below is used in the preparation of ammonia gas in the laboratory
Study it and answer the questions that follow
-
Name the parts labeled a, b and c.
-
What is the meaning of the arrow sign d?
-
What is the function of the calcium oxide?
-
Why has c been tilted downwards?
-
State the method by which the gas is collected.
-
How will you test for the gas?
-
Give the names and the chemical formulae of the compounds that form the content of c.
-
-
In an experiment, a leaf that is partly green and partly yellow is plucked from a tree and the leaf is
I. boiled for a minute
II. dipped in warm alcohol
III. washed in cold water
IV. dipped in iodine solution
One part of the leaf turns blue-black after the dipping in iodine solution while the other part remains unchanged.-
Explain why each of the processes I, II and III is carried out.
-
Which part of the leaf turns blue-black? Explain.
-
Why does the other part of the leaf not change colour?
-
What conclusion can you draw from the experiment?
-